NEW Total Alkalinity Titrator
Measure the ability to absorb protons while maintaining the pH of an aquatic system
Get a Quote
Why Measure Total Alkalinity?
Total alkalinity measurements help researchers investigate the impacts of ocean acidification and climate change on marine ecosystems. As atmospheric CO2 levels rise, oceans act as carbon sinks and absorb excess quantities. This process alters their pH levels and often negatively impacts aquatic life.
By quantifying total alkalinity, a body of water’s buffering capacity against acidification can be assessed and tracked. This helps in predicting future impacts on marine life, including calcifying organisms such as coral reefs and shellfish.
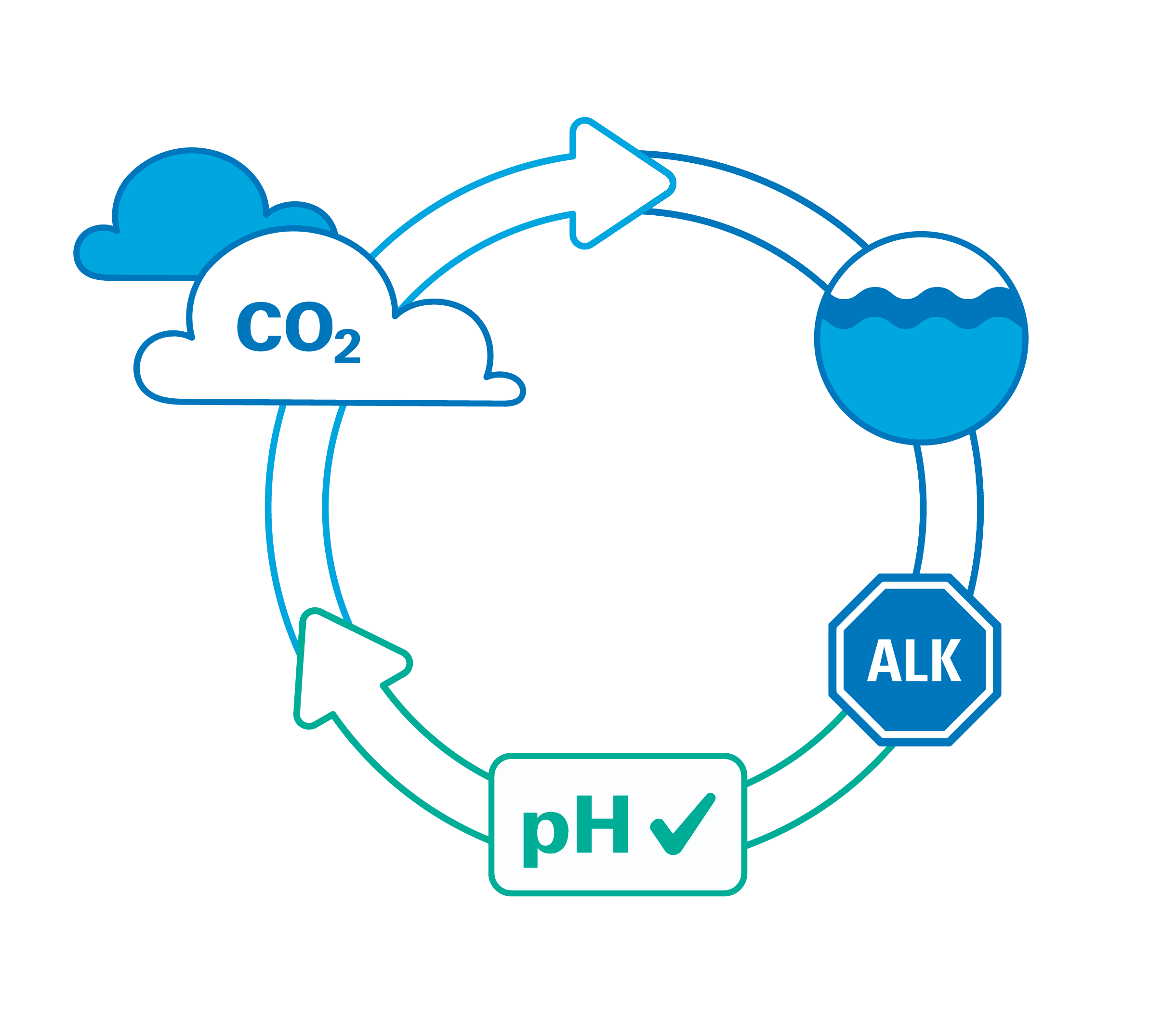
What is Total Alkalinity?
Total alkalinity is the measure of water’s capacity to absorb protons—and neutralize acids—without changing the aquatic system’s pH. Total alkalinity measurements provide insights into the buffering capabilities of a body of water, which is essential for maintaining stable pH levels and supporting aquatic life.
Why Measure Total Alkalinity?
Total alkalinity measurements help researchers investigate the impacts of ocean acidification and climate change on marine ecosystems. As atmospheric CO2 levels rise, oceans act as carbon sinks and absorb excess quantities. This process alters their pH levels and often negatively impacts aquatic life.
By quantifying total alkalinity, a body of water’s buffering capacity against acidification can be assessed and tracked. This helps in predicting future impacts on marine life, including calcifying organisms such as coral reefs and shellfish.
What is Total Alkalinity?
Total alkalinity is the measure of water’s capacity to absorb protons—and neutralize acids—without changing the aquatic system’s pH. Total alkalinity measurements provide insights into the buffering capabilities of a body of water, which is essential for maintaining stable pH levels and supporting aquatic life.

Advantages of the Total Alkalinity Titrator
- Deploy stationary on a ship or on land
- Suited for both freshwater and seawater applications
- Industry-best, high precision measurements
How is it Measured?
Total alkalinity is measured by adding a strong acid titrant, such as hydrochloric acid (HCI), to a water sample just past the pH endpoint. Small amounts of acid titrant are then added, and pH is measured after each addition.
Next, the Total Alkalinity Titrator calculates total alkalinity using the Gran function. This method uses the titration data, including volume and concentration of HCl.

Specifications
General
- Power requirements: 100 to 240 VAC; 50 / 60 Hz
- Precision: ± 0.1% for an alkalinity of ~ 2300 μmol/L
- Accuracy: ± 0.1% for an alkalinity of ~ 2300 μmol/L
- Typical sample volume: 20 – 25 mL per repeat
- Time per sample: 7 – 10 minutes per repeat
- Operating temperature: 15 to 40 °C
- Computer: Windows® 10 OS
- Communication: RS-232 serial with USB adapter (included)
- Operating conditions: Indoor
- Dimension: 28 × 30 × 24 cm (H × W × D)
Specifications subject to change without notice.
References
Cai, W.-J., Wang, Y. and Hodson, R.E. (1998). Acid-Base Properties of Dissolved Organic Matter in the Estuarine Waters of Georgia, USA. 62(3), pp.473–483. doi:https://doi.org/10.1016/s0016-7037(97)00363-3.
Gran, G. (1952). Determination of the equivalence point in potentiometric titrations. Part II. Analyst, [online] 77(920), pp.661–671. doi:https://doi.org/10.1039/AN9527700661.
Dive deeper into your research.
Get a Quote